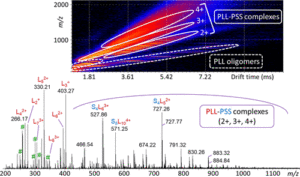
Noncovalent interactions are weak and mostly reversible such as electrostatic or ionic, π-systems, hydrogen bonding, van Der Waals, and hydrophobic interactions. The interaction of molecules with their surroundings is the major fact for designation of their functional characteristics. Secondary and tertiary structures of proteins are regulated by noncovalent interactions so that proteins can attain their specific functions in the dynamic systems of organisms. Conformational changes may alter the interaction behavior of a protein. The conformational characterization of noncovalent complexes is very important issue for obtaining information about their intrinsic characteristics. Studies on conformational characterization of noncovalent complexes provide useful data for understanding the basis of interaction mechanisms in biomolecular recognition, drug or biomolecule delivery systems.
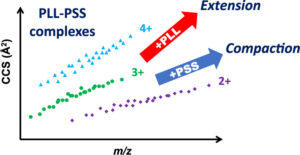
Mass spectrometry enables obtaining highly accurate results for the identification of noncovalent complex structures at high sensitivity. Especially using soft ionization techniques such as Matrix-Assisted Laser Desorption/Ionization (MALDI) and Electrospray Ionization (ESI) provides the analysis of complex structures in their intact forms. It is possible to determine exact mass and isotope patterns that provide identification of elemental compositions of analyte in detail using mass spectrometry. This information can be used to make calculations for determination of stoichiometry of noncovalent complexes. Gas-phase reactions such as fragmentation of complex structures or adduct formation are also monitored. Besides the structural information spatial arrangements of noncovalent complexes can also be defined using different mass spectrometric approaches. Mass spectrometry also provides monitoring the solution phase reactions of noncovalent complexes. In a number of experiments thermochemistry and kinetics of noncovalent complexes in both solution and gas phases are evaluated using mass spectrometric techniques.